Clinical Trials
Two coronavirus vaccines proven safe for human – research
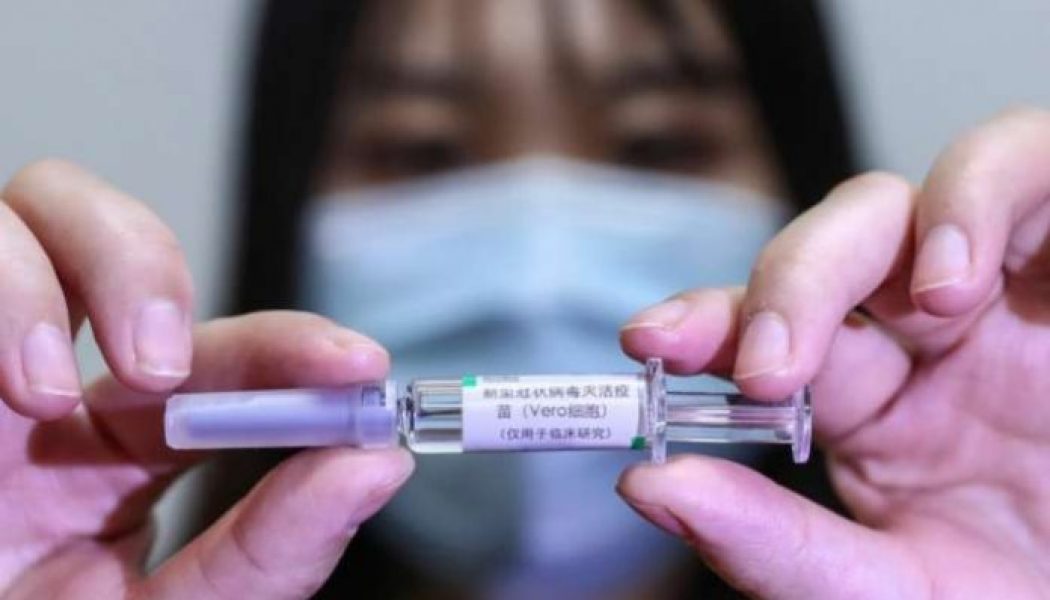
Two coronavirus vaccine have proven safe for humans and produced strong immune reactions among patients involved in separate clinical trials, doctors said Monday. The first trial among more than 1,000 adults in Britain found that the vaccine-induced “strong antibody and T cell immune responses” against the novel coronavirus. A separate trial in China involving more than 500 people showed most had developed widespread antibody immune response. The studies, published in The Lancet medical journal, constitute a major step on the road towards a COVID-19 vaccine that is effective and safe for widespread use. The authors of the studies said that they encountered a few adverse side effects from the vaccine candidates. However, they cautioned that more research was needed, particularly among older...
NAFDAC warns against use of Chloroquine for coronavirus treatment
The National Agency for Food and Drug Administration and Control (NAFDAC) has, again, warned Nigerians against taking Chloroquine as treatment for Coronavirus (COVID-19). “Nobody should buy Chloroquine and use it. If you have COVID-19, go to a doctor. We have warned Nigerians and shall do that again. Do not take Chloroquine,” Mojisola Adeyeye, NAFDAC’s Director General, told newsmen on Thursday in Abuja. “The delay in approving some of the drugs for treatment of the virus is because of the clinical trials the agency is carrying out on them. “So far, no group or individual has proffered any solution to the treatment or management of the pandemic,” she said. Mrs Adeyeye, a professor, said the Chinese government carried out lots of trials on Chloroquine before it approved its use for treatmen...